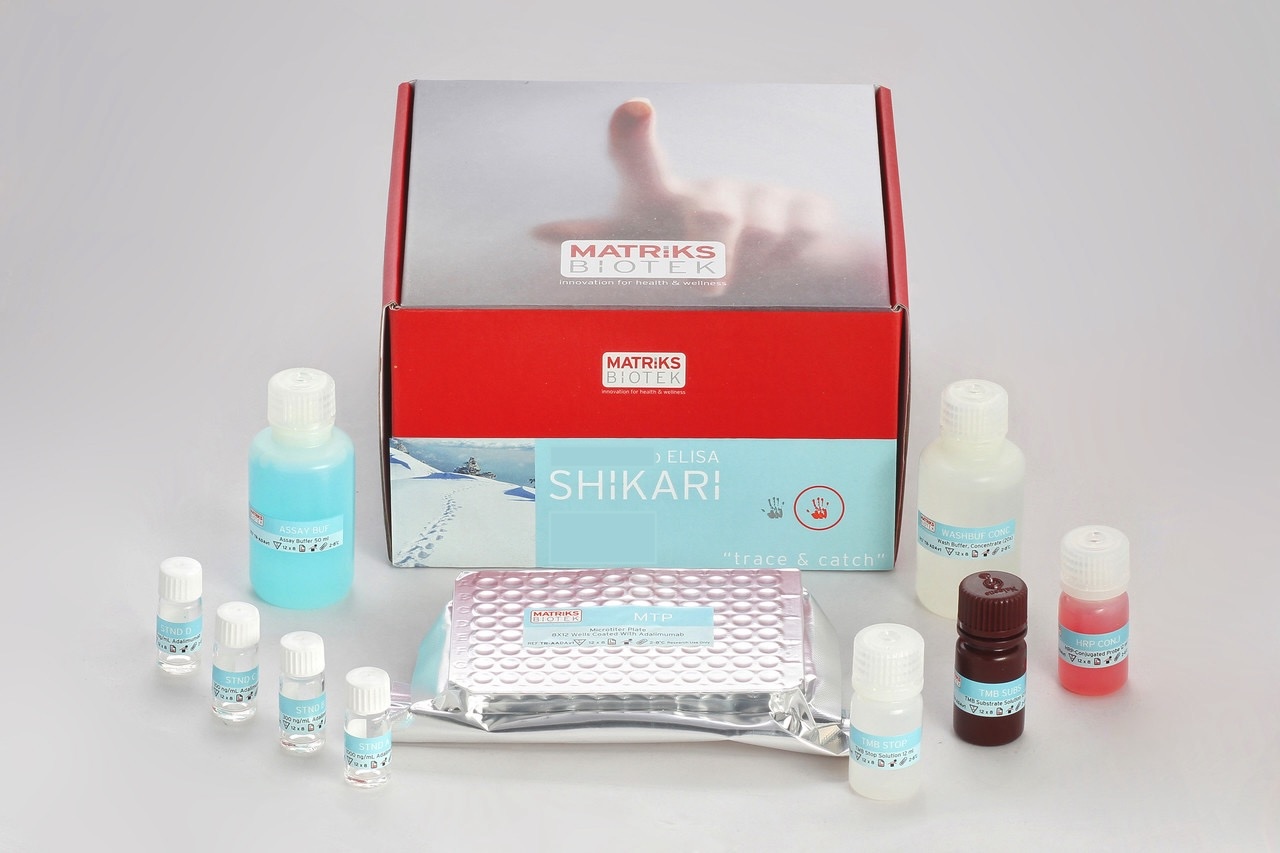
Target Capture Neutralizing Antibodies Immunoassay-Infliximab
Enzyme immunoassay to detect neutralizing antibodies to infliximab in serum and plasma samples
This kit has been developed for detecting ‘Free’ neutralizing antibodies to infliximab. The kit is composed of 4 different controls,
- Positive control: It contains netralizing antibody
- Negative control
- Cut-Off control: Contains certain amounted neutralizing antibody. Corresponse to lower limit of detection (LLOD)
- Non-Neutralizing Antibody Negative Control: Contains non-neutralizing antibodies binding to the drug
With these controls, the kit provides reliable results.
The Cut Off serum contains the LLOD amount and provides accurate true positive or negative results.
A non-neutralising antibody that binds to the drug without affecting the test results can be considered as a negative control.
The kit was also checked with various negative serum samples, including RF(+), CRP(+), ANA(+) sera. All these negative sera did not interfere with the test system. Please refer to the validation report.
We strictly recommend taking sample just before the next dose administration from the patient.
| Required Volume (µl) | 10 |
| Total Time (min) | 140 |
| Sample | Serum, plasma |
| Sample Number | 96 |
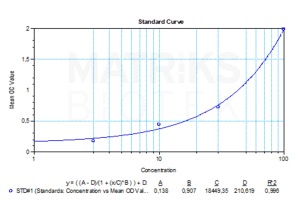
| Detection Limit (ng/mL) | 20 |
| Spike Recovery (%) | – |
| Shelf Life (month) | 6 |
| Assay type | Qualitative |
| Species Reactivity | Human |
| Storage conditions | Store at +4°C. Please refer to protocols. |
| Shipping conditions | At room temperature |



Reviews
There are no reviews yet.