Enzyme immunoassay for the quantitative determination of antibodies to Certolizumab (Cimzia®) in serum and plasma with confirmation.
This kit has been especially developed for the quantitative antibodies to Certolizumab in serum and plasma samples
Certolizumab Drug Bank Accession Number is DB08904.
Certolizumab pegol is a tumor necrosis factor (TNF) blocker used to treat a variety of autoimmune and autoinflammatory conditions like Crohn’s disease, rheumatoid arthritis, active psoriatic arthritis, ankylosing spondylitis, axial spondyloarthritis, and plaque psoriasis. Certolizumab pegol is a pegylated monoclonal antibody against the tumor necrosis factor-alpha (TNF-alpha). It is formed with a humanized Fab fragment of 50 kDa, from an IgG 1 isotype, fused to a 40 kDa polyethylene glycol moiety replacing the Fc antibody region. The absence of the Fc region was ideated to prevent complement fixation and antibody-mediated cytotoxicity as well as to markedly increase its half-life.
All SHIKARI® ELISA kits are suitable for biosimilar work.
| Required Volume (µl) | 5 |
| Total Time (min) | 140 |
| Sample | Serum, plasma |
| Sample Number | 96 |
| Detection Limit (ng/mL) | 5 |
| Spike Recovery (%) | Between 85-115 |
| Shelf Life (year) | 1 |
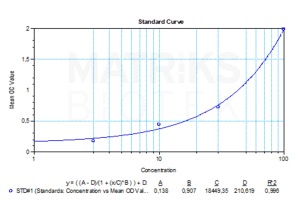
| Assay type | Quantitative |
| Species Reactivity | Human |
| Storage conditions | Store at +4°C. Please refer to protocols. |
| Shipping conditions | At room temperature |



Reviews
There are no reviews yet.