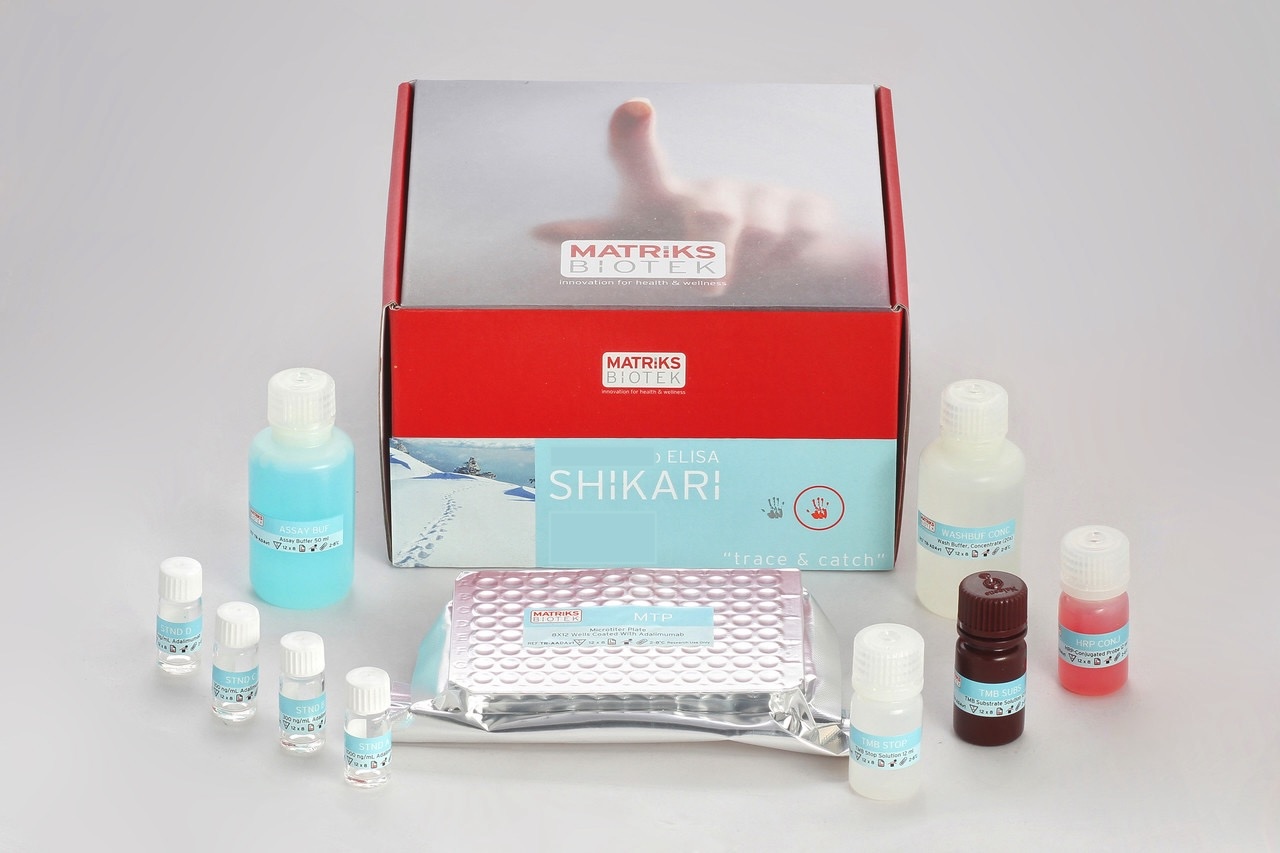
Enzyme immunoassay for the quantitative determination of free Infliximab-biosimilar (Remsima®) in serum and plasma
This kit has been especially developed for the quantitative determination of infliximab-biosimilar (Remsima®) in serum and plasma samples between the Cmin and Cmax range of concentrations.
Infliximab Drug Bank Accession Number is DB00065.
Infliximab is a monoclonal anti-tumor necrosis factor alpha antibody used in the treatment of a wide variety of inflammatory conditions such as rheumatoid arthritis, Crohn’s disease and ankylosing spondylitis. Infliximab binds with high affinity to soluble and transmembrane forms of TNF-α to disrupt the proinflammatory cascade signaling. Binding of antibodies to TNF-a prevents TNF-a from interacting with its receptors and helps the patient recover. Measurement of biological drug trough levels and antibody to biological drug gained high importance during the course of treatment. These measurements enable dose adjustments and switch to another class of biological drug when necessary.
Remsima®,the world first biosimilar mAb (approved in 2013 by EMA). The Agencys Committee for Medicinal Products for Human Use (CHMP) decided that, in accordance with EU requirements, Remsima® has been shown to have a comparable quality, safety and efficacy profile to Remicade®.
All SHIKARI® ELISA kits are suitable for biosimilar work.
| Required Volume (µl) | 10 |
| Total Time (min) | 70 |
| Sample | Serum, plasma |
| Sample Number | 96 |
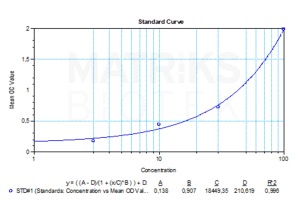
| Detection Limit (ng/mL) | 10 |
| Spike Recovery (%) | Between 85-115 |
| Shelf Life (year) | 1 |
| Assay type | Quantitative |
| Species Reactivity | Human |
| Storage conditions | Store at +4°C. Please refer to protocols. |
| Shipping conditions | At room temperature |



Reviews
There are no reviews yet.