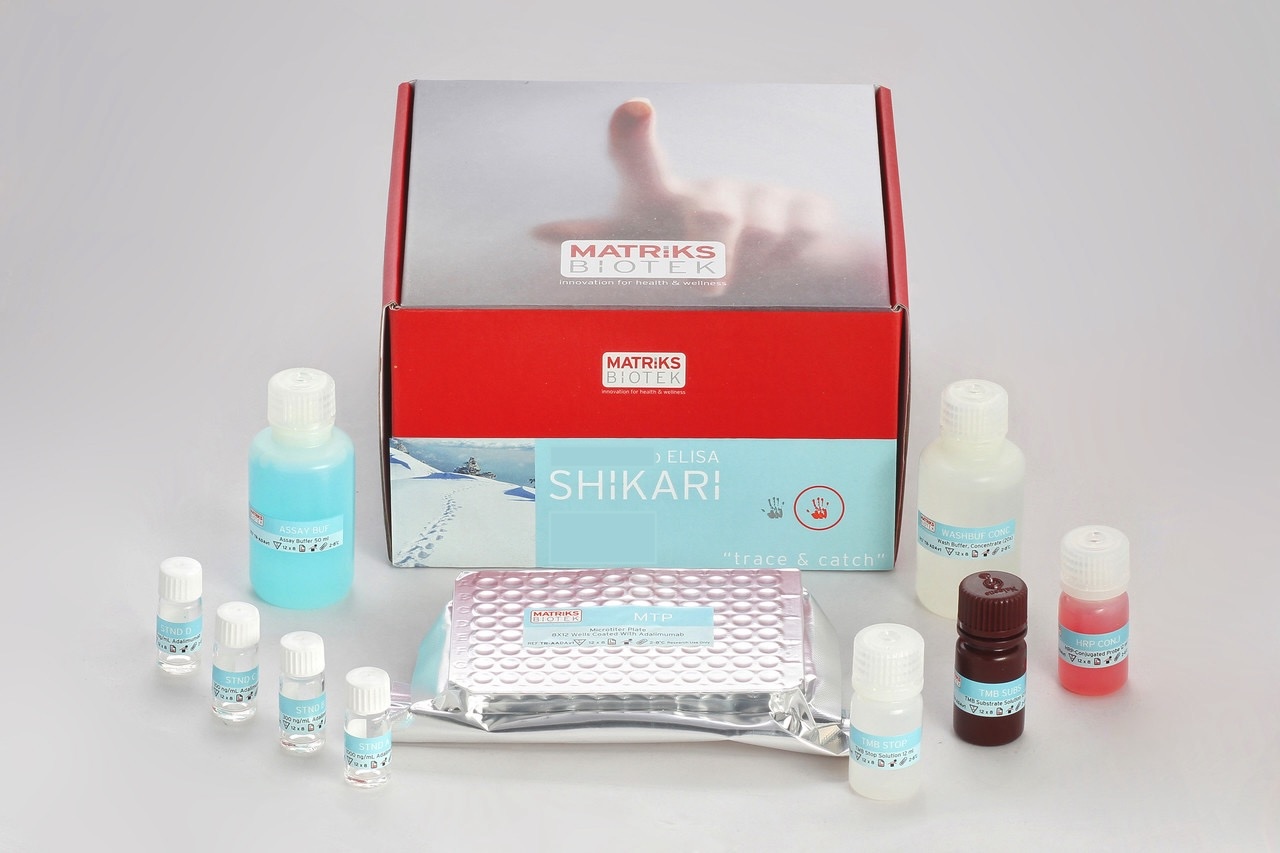
Enzyme immunoassay for the quantitative determination of antibodies to Infliximab (Remicade®) in serum and plasma with confirmation.
This kit has been especially developed for the quantitative antibodies to infliximab in serum and plasma samples
Infliximab (Remicade) was associated to the development of anti-Infliximab antibodies, even some were reported to be neutralizing, in various percentages of patients during therapy with the drug Remicade®. This might lead to severe complications. This kit can be efficiently used for monitoring anti-Infliximab antibodies during therapy and offers the clinician a tool for decision on possible preventive measures such as possible addition of immunosuppressive drug to reduce anti-Infliximab antibodies.
All SHIKARI® ELISA kits are suitable for biosimilar work.
| Required Volume (µl) | 20 |
| Total Time (min) | 140 |
| Sample | Serum, plasma |
| Sample Number | 96 |
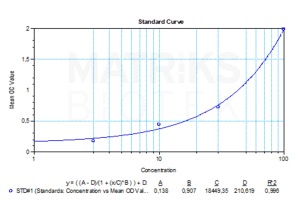
| Detection Limit (ng/mL) | 3,125 |
| Spike Recovery (%) | Between 85-115 |
| Shelf Life (year) | 1 |
| Assay type | Quantitative |
| Species Reactivity | Human |
| Storage conditions | Store at +4°C. Please refer to protocols. |
| Shipping conditions | At room temperature |



Reviews
There are no reviews yet.