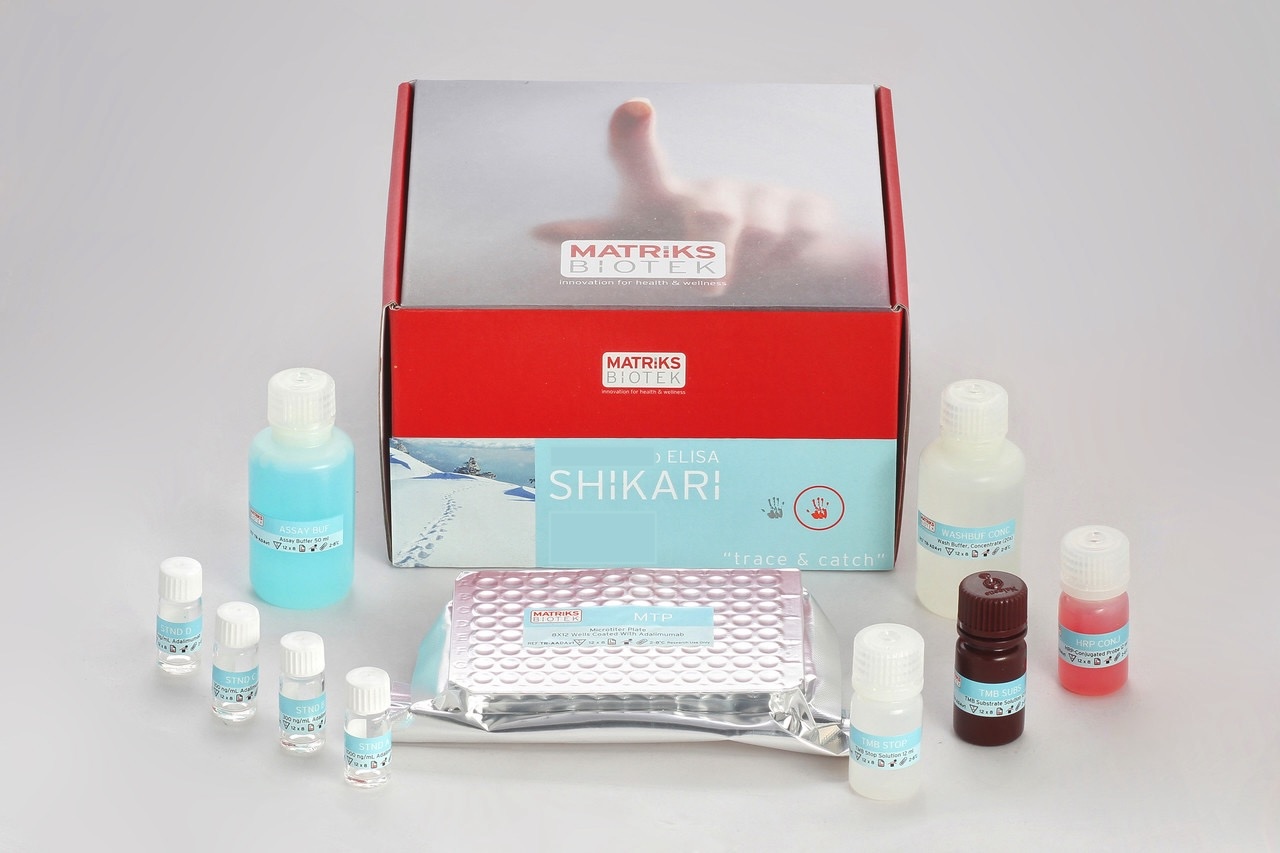
Enzyme immunoassay for the quantitative determination of Guselkumab (Tremfya®) in human serum and plasma
This kit has been especially developed for the quantitative determination of Guselkumab in serum and plasma samples between the Cmin and Cmax range of concentrations.
Guselkumab Drug Bank Accession Number is DB11834
Guselkumab, a monoclonal antibody of the human immunoglobulin G1 lambda (IgG1λ) class, specifically targets the p19 subunit of interleukin-23 (IL-23), a key inflammatory cytokine involved in activating the CD4+ T-helper (Th17) cell pathway responsible for inducing psoriatic plaque formation. In clinical trials, guselkumab has demonstrated efficacy in improving skin clearance and relieving symptoms of psoriasis by blocking IL-23’s interaction with its receptor on dendritic cells and keratinocytes. Measurement of biological drug trough levels and antibody to biological drug gained high importance during the course of treatment. These measurements enable dose adjustments and switch to another class of biological drug when necessary.
All SHIKARI® ELISA kits are suitable for biosimilar work.
| Required Volume (µl) | 10 |
| Total Time (min) | 70 |
| Sample | Serum, plasma |
| Sample Number | 96 |
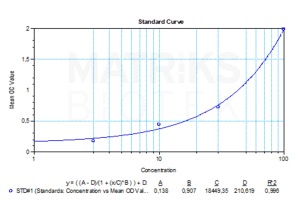
| Detection Limit (ng/mL) | 0,625 |
| Spike Recovery (%) | Between 85-115 |
| Shelf Life (year) | 1 |
| Assay type | Quantitative |
| Species Reactivity | Human |
| Storage conditions | Store at +4°C. Please refer to protocols. |
| Shipping conditions | At room temperature |



Reviews
There are no reviews yet.