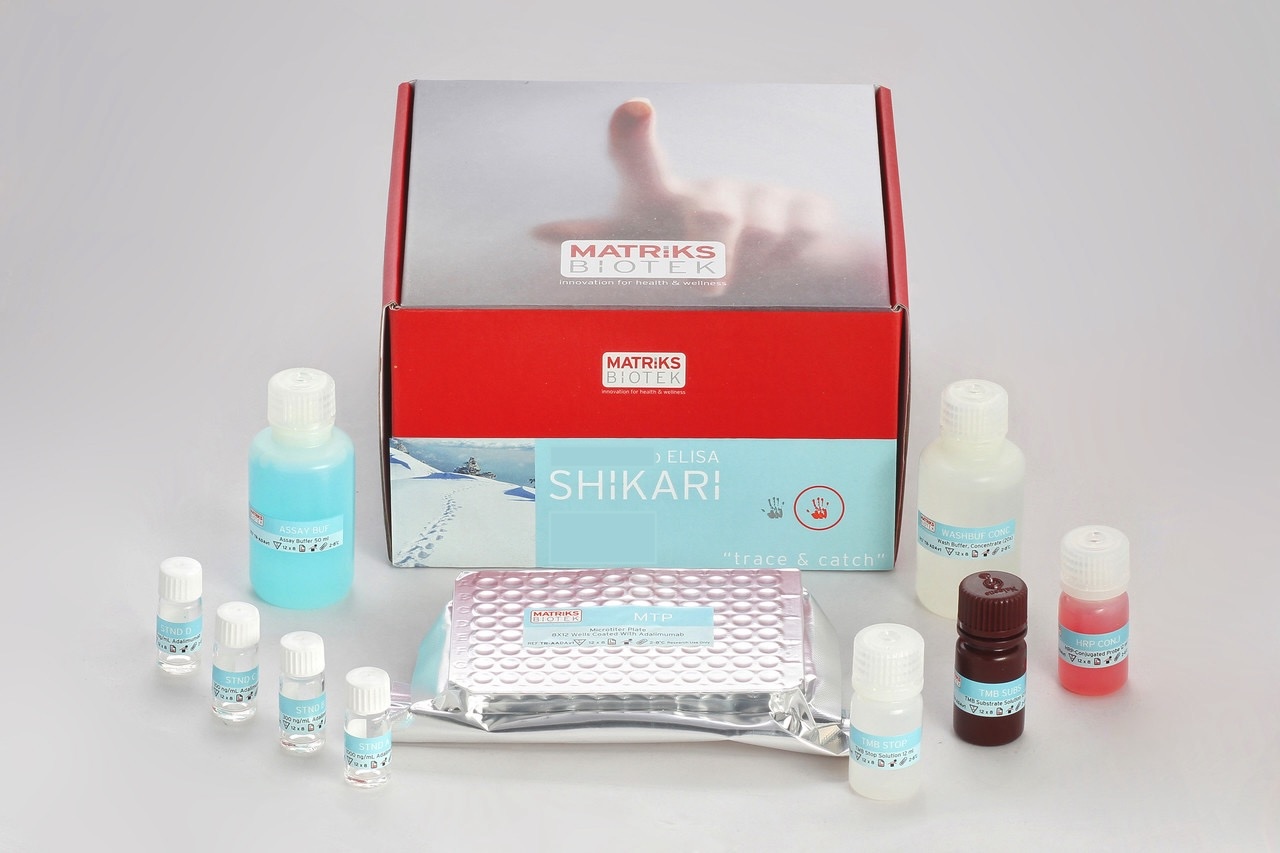
Enzyme immunoassay for the qualitative determination of antibodies to Eculizumab (Soliris®) in serum and plasma.
This kit has been especially developed for the qualitative determination of antibodies to eculizumab in serum and plasma samples.
Eculizumab (Soliris®) was associated to the development of anti-Eculizumab antibodies, even some were reported to be neutralizing, in various percentages of patients during therapy with the drug Soliris®. This might lead to severe complications. This kit can be efficiently used for monitoring anti-Eculizumab antibodies during therapy and offers the clinician a tool for decision on possible preventive measures such as possible addition of immunosuppressive drug to reduce anti-Eculizumab antibodies.
All SHIKARI® ELISA kits are suitable for biosimilar work.
| Required Volume (µl) | 20 |
| Total Time (min) | 140 |
| Sample | Serum, plasma |
| Sample Number | 96 |
| Detection Limit (ng/mL) | +/- |
| Spike Recovery (%) | – |
| Shelf Life (year) | 1 |
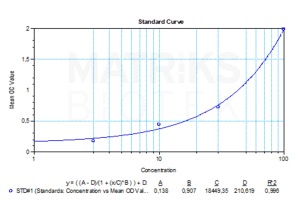
| Assay type | Qualitative |
| Species Reactivity | Human |
| Storage conditions | Store at +4°C. Please refer to protocols. |
| Shipping conditions | At room temperature |



Reviews
There are no reviews yet.