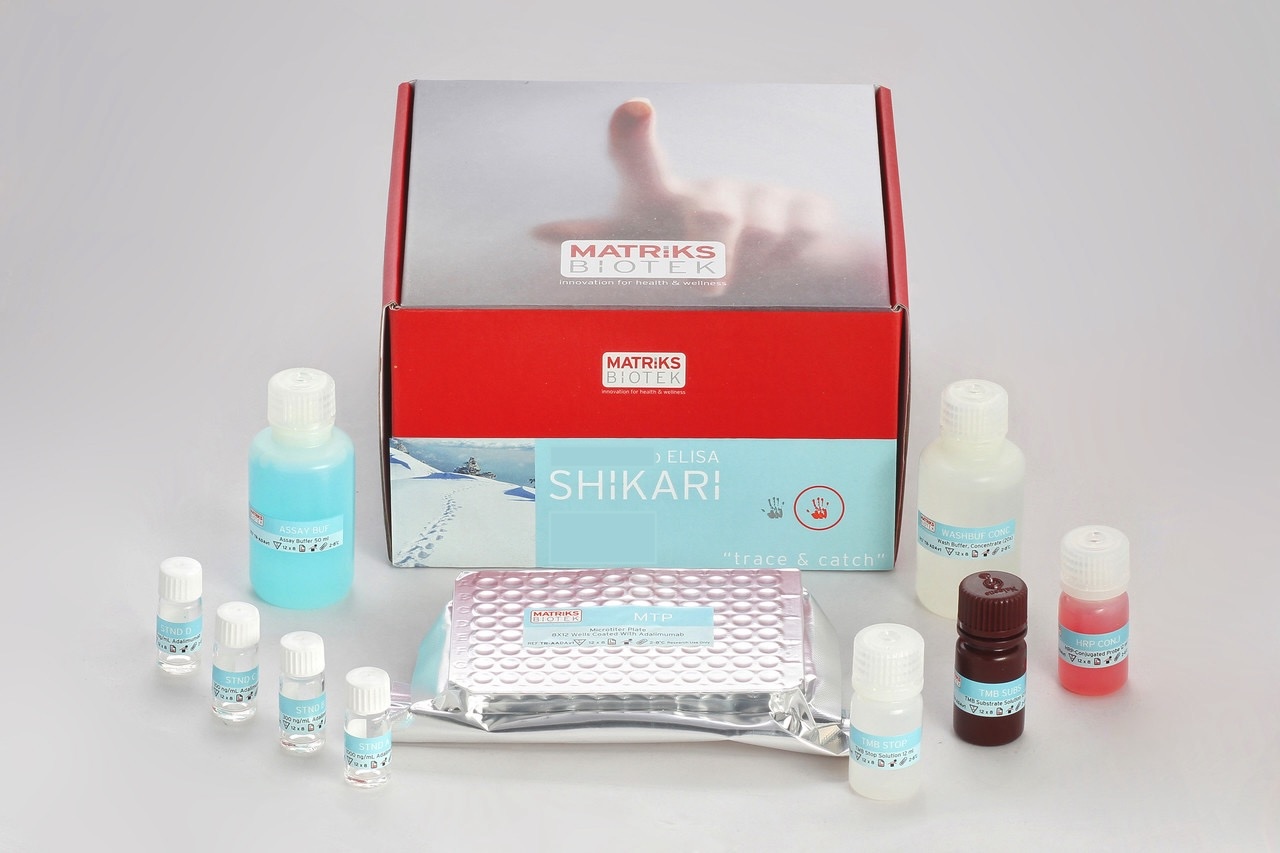
Enzyme immunoassay for the quantitative determination of antibodies to Bevacizumab (Avastin®) in serum and plasma with confirmation.
This kit has been especially developed for the quantitative antibodies to Bevacizumab in serum and plasma samples
Bevacizumab (Avastin®) was associated to the development of anti-bevacizumab antibodies, even some were reported to be neutralizing, in various percentages of patients during therapy with the drug Avastin®.This might lead to severe complications. This kit can be efficiently used for monitoring anti- bevacizumab antibodies during therapy and offers the clinician a tool for decision on possible preventive measures such as possible addition of immunosuppressive drug to reduce anti-bevacizumab antibodies.
All SHIKARI® ELISA kits are suitable for biosimilar work.
| Required Volume (µl) | 20 |
| Total Time (min) | 140 |
| Sample | Serum, plasma |
| Sample Number | 96 |
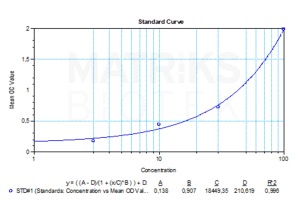
| Detection Limit (ng/mL) | 7,5 |
| Spike Recovery (%) | Between 85-115 |
| Shelf Life (year) | 1 |
| Assay type | Quantitative |
| Species Reactivity | Human |
| Storage conditions | Store at +4°C. Please refer to protocols. |
| Shipping conditions | At room temperature |



Reviews
There are no reviews yet.